Data requests
Accessing APSI data for research is only possible following approval. All data held by APSI is de-identified.
CURRENT DATA AVAILABILITY UNDER APSI
The eventual goal for APSI is to create a national database of all calls to Australian PICs. The four Australian PICs will join APSI with a staged approach, once legal and governance requirements have been met. At present, NSWPIC data is available for request from APSI. NSWPIC takes approximately 50% of the nation’s poisoning calls, including a sample of calls from all states and territories. This allows researchers to request NSWPIC data while the APSI team works on integrating the other PICs’ datasets.
DATA REQUESTS AND GOVERNANCE OVERVIEW
All data held and released by APSI is de-identified. We provide two types of data release to eligible applicants: aggregate (summary) datasets and unit record releases (case listings). Each type of release has access rules and all requests must undergo the APSI governance process.
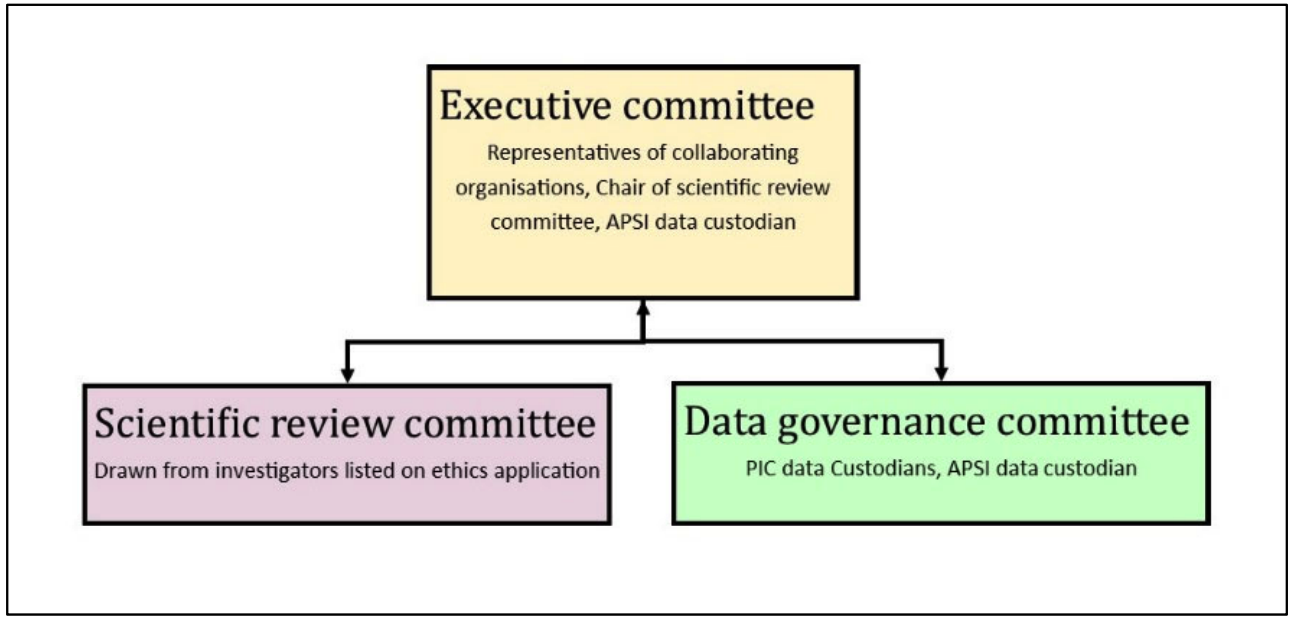
APSI is managed by three main committees:
-
- Executive committee: responsible for APSI governance oversight.
- Scientific review committee: comprising of APSI investigators, the scientific review committee will assess submitted research protocols to ensure the project is well designed and falls under the research objectives of APSI.
- Data governance committee: comprised the PIC data custodians and the APSI data custodian (or delegate/s). The data governance committee makes the final decision about data release.
Ethics Coverage
Simple requests involving aggregated data will be released to all applicants under the standing HREC approval in line with the APSI protocol: Access to aggregated data [standing approval (ii)].
To be covered by the APSI HREC approval for unit record data (case listing) requests need to meet the following criteria:
- Involves retrospective analysis of APSI data
- There are no attempts at re-identification of the data
- Counts <5 will be suppressed if there is potential for re-identification
- Does not involve linking of the data from the APSI dataset with data from other sources
- Is led by an investigator listed on the APSI protocol or is a staff member/formal affiliate of an APSI investigator’s institution and has been endorsed for access to the dataset by that investigator
- Has undergone APSI scientific review and received approval from the APSI data governance committee
Requests that do not meet the above criteria will need to provide evidence of HREC approval prior to data release. HREC approvals are not required at the time of submitting the request for initial assessment of eligibility.
CONDITIONS OF USE
All data releases will be subject APSI conditions of use and publication policy (sections 8 and 9 of the APSI data governance and access policy). All releases will occur after appropriate data governance is in place including a data transfer agreement or another contractual arrangement, if required. Applicants should consult the APSI data manager about governance requirements.
Request process
Requests are submitted using the electronic APSI data request form. The data request procedure is described in section 10 of the APSI data governance and access policy.
Researchers are strongly advised to consult the APSI documents including data dictionaries and data access policy to ensure compliance with data release policies and feasibility of their studies. Researchers are advised to contact the APSI data manager prior to submitting a unit record (case listings) data request.
INDEPENDENT REVIEW
Depending on the nature of your request, your application for data will be reviewed by the APSI Scientific Committee and/or the APSI Data Governance Committee. More detailed requests will require Scientific Committee review of rationale and methods (including a statistical analysis plan). As part of this review process, you may be asked for additional information or clarification.
Following approval, the APSI data manager will contact you about the mechanism of data provision.
Referencing this dataset
Below is the suggested format for referencing the APSI databank:
Cairns R, Buckley NA, Dzidowska M, Benger L, Roberts DM, Wright NE, Wylie C, Elliott RE, Greene S, Heiner M, Armstrong J, Merwood N, Isoardi KZ, Chiew AL, Brown JA, Dawson AH, on behalf of the APSI Investigators, 2024. Australian Poisoning Surveillance Initiative Databank [dataset]. Metadata record available from: https://ses.library.usyd.edu.au/handle/2123/33312